
I was first introduced to the notion of pH and skincare by one of my favorite Youtube (YT) channels Hot and Flashy. Angie, the You Tuber, is an over 50, science nerd, and anti-aging aficionado after my own heart. As I watched her video, my brain was saying “Whoa, whoa, whoa – back up there a minute. I need more information!”
Welcome to how my brain works and gets me stuck in curiosity-fueled rabbit holes.
If you’re a dork-a-potamus-rex like me (I may be the only one) then you probably already read last week’s post about Acids and Alkalis. This was meant to provide a foundation for this article. If you want to go peruse it first, we’ll still be here when you’re done.
Skin’s pH
Come to find out, healthy skin is slightly acidic. In fact, baby’s skin is more neutral when they are born and it becomes more acidic over time. As we age (I mean “gain wisdom”) our skin can become more alkaline. This is why we care about pH in our pursuit of anti-aging. The products we use (and apparently the foods we eat – a different rabbit hole for a different time) can impact our skin’s pH.
Our skin is protected by something referred to as the acid mantle that basically creates a barrier between our skin and the external world. It keeps moisture in and bad things like environmental pollutants and bacteria out. When it’s working well, we have beautiful skin. When it’s not, we may have dry skin, dermatitis, rosacea, and other skin conditions.
Before you get obsessed, the good news is that we don’t need to be heavily involved in repair our skin’s acidity or acid mantle. Basically we just have to get out of the way. That means, don’t use products that are making it work harder or that are irritating to it.
But then that leads to the next obvious question, how do we know what falls into THAT category?
That, dear ones, is exactly what got me on this path in the first place. Nerd Alert!
I started reading articles and scientific papers about product absorption and pH.
There is simultaneously a lot of information and not a lot of detail. Everyone agrees that pH matters, but there isn’t anyone putting together the big picture. I walked away with almost as many questions as I had when I started.
Does the order of skincare matter from a pH perspective? According to Paula’s Choice, the answer is no.
When products support one another (like vitamin C, vitamin E, and ferulic acid) it seems to be through some chemical process and has no mention of pH. Specifically ferulic acid boosts UV protection of vitamin C and vitamin E. Vitamin C coupled with vitamin E regenerates it when it oxidizes (is exposed to oxygen).
Where does that leave us?
I landed on three takeaways:
- Skin’s pH is mostly self-regulating
- Some products have an optimal absorption pH
- Focus on the products only if they matter to me (for impact or cost)
Testing pH
The two products I decided to focus on, largely because it was easier to find information about pH mattering, is Vitamin C and mineral sunscreen. They both have optimal pH windows for effectiveness. Vitamin C has an optimal window for absorption and zinc oxide/titanium oxide have optimal windows for UV protection.
Vitamin C
Vitamin C absorbs best at a pH around 3.5. If you have sensitive skin, you may fare better with a slightly less acidic vitamin C with a pH of 5 or 6. It won’t absorb as well, but it will still benefit you.
Or that’s what all of the “experts” say…Ready for some critical thinking?
As I was researching this, I was able to find abundant sources to provide this “rule” but I had a heck of a time getting to the original scientific study. It appears that this “rule” stems from a single study done on pig’s skin. I’m pragmatically less bothered by the pig’s skin (anti-vivisection all the way but what’s done is done) and more focused on the method. The skin was SOAKED in the vitamin C solutions for 24 hours in a controlled manner that prevented evaporation. This immediately makes the translation of results to OUR application (topical product on human skin) suspect, right?
I was able to find a lot more studies on the optimal concentration of vitamin C (15-20% if you can tolerate it). There is also evidence that different formulations of vitamin C (as well as different forms) affect the skin differently – specifically with heightened or reduced reaction and inflammation.
My personal conclusion is, there is a reasonable correlation between vitamin C, pH, and potency that impacts how much our skin likes it. However I wouldn’t go so far as to call this a rule. More like an intriguing data point that could be more interesting if there was more scientific validation of it in human skin.
If there are formulas your skin likes and you’re curious like me, you can buy a simple test kit to see where it falls in the pH range.
Make sure you pay attention to the range you can test.
I have two kits one tests .5 to 5.5 (acidity) and the other tests 4.5 to 9.0 (acidity to alkalinity).
I had products, I had test strips so I figured…I might as well see what happens.
The “Science”
I started with three forms of vitamin C. NIOD Ethylated L-Ascorbic Acid 20% network (anhydrous solution), Good Genes Vitamin C Booster Powder (powdered vitamin C), and Paula’s Choice C15 Super Booster (normal formulation).
Vitamin C is water soluble so the most common form is the water based. The only downside is that it can oxidize and lose effectiveness. Non-water or anhydrous solutions have the benefit of being more stable and not oxidizing but then you have to figure out if the formula base causes problems for you. (I really enjoy the NIOD version.) Powdered vitamin C is ultra stable. You add it to your products just before you use it. But you have to be careful not to use too much and mix it well with your base product. I think I trust product chemists more than myself.

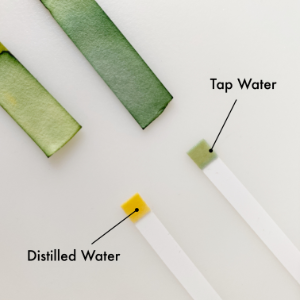
Baseline: Tap water and distilled water 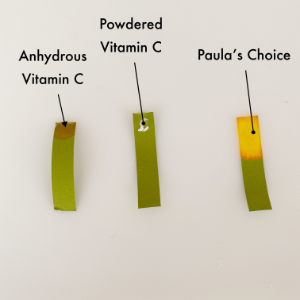
Each product on its own 
Products with a drop of tap water
pH requires WATER for its measurement. You may need to add a drop of water to get it to register.
Observations
I had two different test kits. The green paper version measured pH from 0.5 to 5.5. Basically that was only going to work for testing acidity. The thinner strips with just the strip at the end tests pH from 4.5 to 9.0 so basically the more neutral to acid range. (Remember that water is theoretically neutral and skin’s natural pH is around 5.5.)
I was originally going to use distilled water but discovered that it tends to be a little less neutral (and doesn’t have the ions to help it register well on test strips). The tap water was closer to neutral so that was my water addition. The middle photo shows what the ends of the strip look like with JUST the product. Paula’s Choice immediately registered in the target range of 3.5. Once I added a single drop of water to the end of each, it really started to show the acidity level. The powdered vitamin C ended up where our skin likes it though it seems to lean a little more towards acidity than Paula’s Choice. The NIOD Ethylated L-Ascorbic Acid reads like it’s a bit more acidic. Honestly given the base that it’s in, I’m not sure it’s accurate.
My conclusion? It’s fun to make paper change color but in truth the range of variables can’t be well controlled. I’ll stick with what I like best which is Paula’s Choice…oxidation aside. On to sunscreen…
Mineral Sunscreen
Mineral sunscreens are also known as physical sunscreens and they are usually made up of zinc oxide and/or titanium oxide. As I was researching this, I discovered that these two minerals have optimal pH levels. They are alkaline and should have a pH of at least 7.1. Any lower and the oxide starts to dissolve which means it is less effective. The “experts” recommend that you find a pH-balanced mineral sunscreen. The problem is, apparently that’s not a thing that our sunscreen manufacturers routinely do. Paula’s Choice reveals they balance the pH in their sunscreens, but it’s not on the bottle. You have to go hunting for it.
The other note here is that since it’s more alkaline than our normal, healthy skin, we definitely don’t want to leave it on overnight. Definitely get it off your skin before you sleep and allow your skin to rebalance its pH.
The “Science”
I grabbed four mineral sunscreens I have hanging around and decided to check them out. I like them all. From left to right they are: Drunk Elephant Umbra™ Sheer Physical Defense SPF 30 (20% Zinc Oxide), Farmacy Green Defense Daily Mineral Sunscreen SPF 30 (Titanium Dioxide 2.4%, Zinc Oxide 5.82%), Murad City Skin Age Defense Mineral Sunscreen SPF 50 (Titanium Dioxide 2.7%, Zinc Oxide 10.0%), and elta MD UV Physical Sunscreen SPF 41 (Zinc oxide 9.0%, Titanium dioxide 7.0%).
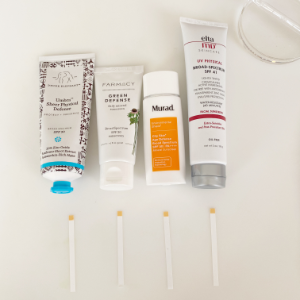

Just product on the strips 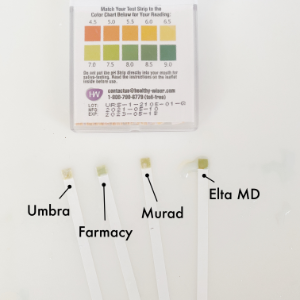
Product with a drop of tap water
Observations
First observation…my photos suck. Second observation, I mislabeled the Drunk Elephant “Umbra” but didn’t have time to fix it. This month’s motto has been “roll with the punches”. When the product alone was put on, the strips weren’t reactive. However, after I add a drop of tap water, they started to change. Do I conclude it worked? As before, there are variables that can’t easily be controlled in a home setting. I don’t even know if the strips actually work or if I approached it properly. If I did (that’s a big if) then the products all seem to fit into the generally pH of 7.1 or higher. So it worked…or at least LOOKS like it did.
The Bottom Line
I find all of this fascinating, but let’s get practical for a minute. What are you supposed to do with all of this newfound knowledge?
Nothing.
This is interesting brain food that will make you smarter and help anti-age your brain. Your own takeaway is much simpler. Just pay attention. Listen to your skin. Be gentle to it. Use products which are balanced.
Feel free to test them with my totally unscientific science-y approach.
The companies creating these products, for the most part, are paying attention to this pH stuff for us.
However, if you experience issues with specific products or formulations OR if you’re just teeming with boundless curiosity like me, you might try testing your product’s pH. And if everything is working well in your skincare world or you simply can’t give it anymore brain space than reading this blog, I won’t be offended. At least you got a little smarter.